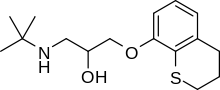
Tertatolol
Tertatolol (Artex, Artexal, Prenalex) is a medication in the class of beta blockers, used in the treatment of high blood pressure. It was discovered by the French pharmaceutical company Servier and is marketed in Europe.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| ECHA InfoCard | 100.073.179 |
| Chemical and physical data | |
| Formula | C16H25NO2S |
| Molar mass | 295.44 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Tertatolol has also been shown to act as a serotonin 5-HT1A and 5-HT1B receptor antagonist, similarly to propranolol and pindolol.[2][3][4]
See also
References
- Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- Langlois M, Brémont B, Rousselle D, Gaudy F (1993). "Structural analysis by the comparative molecular field analysis method of the affinity of beta-adrenoreceptor blocking agents for 5-HT1A and 5-HT1B receptors". Eur. J. Pharmacol. 244 (1): 77–87. doi:10.1016/0922-4106(93)90061-d. PMID 8093601.
- Jolas T, Haj-Dahmane S, Lanfumey L, et al. (May 1993). "(-)Tertatolol is a potent antagonist at pre- and postsynaptic serotonin 5-HT1A receptors in the rat brain". Naunyn-Schmiedeberg's Archives of Pharmacology. 347 (5): 453–63. doi:10.1007/bf00166735. PMID 7686633. S2CID 22129922.
- Soudijn, W.; Olivier, Berend; Wijngaarden, I. van (1997). Serotonin receptors and their ligands. Amsterdam: Elsevier. ISBN 0-444-82041-8.
| β, non-selective | |
|---|---|
| β1-selective | |
| β2-selective | |
| α1- + β-selective | |
| |
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.