Carteolol
Carteolol is a non-selective beta blocker used to treat glaucoma.
 | |
| Clinical data | |
|---|---|
| Trade names | Ocupress |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a601078 |
| License data | |
| Routes of administration | Eye drops |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Metabolism | Liver, active with 8-hydrocarteolol |
| Elimination half-life | 6–8 hours |
| Excretion | Kidney (50-70%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
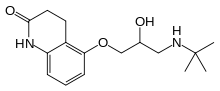
| Formula | C16H24N2O3 |
| Molar mass | 292.379 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
It has been found to act as a serotonin 5-HT1A and 5-HT1B receptor antagonist in addition to being a beta blocker.[1]
It was patented in 1972 and approved for medical use in 1980.[2]
Brand names
Brand names include Cartrol, Ocupress, Teoptic, Arteolol, Arteoptic, Calte, Cartéabak, Carteol, Cartéol, Cartrol, Elebloc, Endak, Glauteolol, Mikelan, Poenglaucol, and Singlauc.
References
- Langlois M, Brémont B, Rousselle D, Gaudy F (1993). "Structural analysis by the comparative molecular field analysis method of the affinity of beta-adrenoreceptor blocking agents for 5-HT1A and 5-HT1B receptors". Eur. J. Pharmacol. 244 (1): 77–87. doi:10.1016/0922-4106(93)90061-d. PMID 8093601.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 460. ISBN 9783527607495.
External links
- El-Kamel A, Al-Dosari H, Al-Jenoobi F (2006). "Environmentally responsive ophthalmic gel formulation of carteolol hydrochloride". Drug Deliv. 13 (1): 55–9. doi:10.1080/10717540500309073. PMID 16401594. S2CID 30222292.
- Kuwahara K, Oizumi N, Fujisawa S, Tanito M, Ohira A (2005). "Carteolol hydrochloride protects human corneal epithelial cells from UVB-induced damage in vitro". Cornea. 24 (2): 213–20. doi:10.1097/01.ico.0000141232.41343.9d. PMID 15725891. S2CID 20523541.
- Trinquand C, Romanet J, Nordmann J, Allaire C (2003). "[Efficacy and safety of long-acting carteolol 1% once daily. A double-masked, randomized study]". J Fr Ophtalmol. 26 (2): 131–6. PMID 12660585.
| α1 |
| ||||
|---|---|---|---|---|---|
| α2 |
| ||||
| β |
| ||||
| |||||
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.