tert-Butyllithium
tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon molecules, including benzene. tert-Butyllithium is available commercially as hydrocarbon solutions; it is not usually prepared in the laboratory. Its synthesis was first reported by R. B. Woodward in 1941.[1]
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
tert-Butyllithium | |
| Identifiers | |
3D model (JSmol) |
|
| 3587204 | |
| ChemSpider | |
| ECHA InfoCard | 100.008.939 |
| EC Number |
|
PubChem CID |
|
| UN number | 3394 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| LiC 4H 9 | |
| Molar mass | 64.055 g mol−1 |
| Appearance | Colorless solid |
| Density | 660 mg cm−3 |
| Boiling point | 36 to 40 °C (97 to 104 °F; 309 to 313 K) |
| Reacts | |
| Acidity (pKa) | 45–53 |
| Hazards | |
| GHS labelling: | |
     | |
| Danger | |
| H225, H250, H260, H300, H304, H310, H314, H330, H336, H411 | |
| P210, P222, P223, P231+P232, P370+P378, P422 | |
| NFPA 704 (fire diamond) | |
| Flash point | −6.6 °C (20.1 °F; 266.5 K) |
| Related compounds | |
Related compounds |
n-Butyllithium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure and bonding
Like other organolithium compounds, tert-butyllithium is a cluster. Whereas n-butyllithium exists both as a hexamer and a tetramer, tert-butyllithium exists as tetramer with a cubane structure. Bonding in organolithium clusters involves sigma delocalization and significant Li−Li bonding.[2]
The lithium–carbon bond in tert-butyllithium is highly polarized, having about 40 percent ionic character. The molecule reacts like a carbanion, as is represented by these two resonance structures.[3] (Given the polarity calculations on the C−Li bond, the "real" structure of a single molecule of t-butyllithium is likely a near-average of the two resonance contributors shown, in which the central carbon atom has a ~50% partial negative charge while the lithium atom has a ~50% partial positive charge.)
Chemical properties
Similar to n-butyllithium, tert-butyllithium can be used for the exchange of lithium with halogens and for the deprotonation of amines and activated C−H compounds.
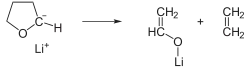
This compound and other alkyllithium compounds are known to react with ether solvents; the half-life of tert-butyllithium is 60 minutes at 0 °C in diethyl ether, 40 minutes at −20 °C in tetrahydrofuran (THF),[4] and about 11 minutes at −70 °C in dimethoxyethane.[5] In this example, the reaction of tert-butyllithium with (THF) is shown:
To minimize degradation by these solvents, reactions involving tert-butyllithium are often conducted at very low temperatures in special solvents, such as the Trapp solvent mixture.
Safety
tert-butyllithium is a pyrophoric substance, meaning that it spontaneously ignites on exposure to air. Air-free techniques are important so as to prevent this compound from reacting violently with oxygen and moisture:
- t-BuLi + O2 → t-BuOOLi
- t-BuLi + H2O → t-BuH + LiOH
The solvents used in common commercial preparations are themselves flammable. While it is possible to work with this compound using cannula transfer, traces of tert-butyllithium at the tip of the needle or cannula may catch fire and clog the cannula with lithium salts. While some researchers take this "pilot light" effect as a sign that the product is "fresh" and has not degraded due to time or improper storage/handling, others prefer to enclose the needle tip or cannula in a short glass tube, which is flushed with an inert gas and sealed at each end with septa.[6] Serious laboratory accidents involving tert-butyllithium have occurred. For example, in 2008 a staff research assistant, Sheharbano Sangji, in the lab of Patrick Harran[7] at the University of California, Los Angeles, died after being severely burned by a fire ignited by tert-butyllithium.[8][9][10]
Large-scale reactions may lead to runaway reactions, fires, and explosions when tert-butyllithium is mixed with ethers such as diethyl ether, and tetrahydrofuran. The use of hydrocarbon solvents may be preferred.
References
- Bartlett, Paul D.; C. Gardner Swain; Robert B. Woodward (1941). "t-Butyllithium". J. Am. Chem. Soc. 63 (11): 3229–3230. doi:10.1021/ja01856a501.
- Elschenbroich, C. "Organometallics" (2006) Wiley-VCH: Weinheim. ISBN 978-3-527-29390-2
- Organometallic reagents: sources of nucleophilic carbon for alcohol synthesis. K. P. C. Vollhardt, N. E. Schore: Organic Chemistry : Structure And Function. 3rd edition, 1999, §8.7.
- Stanetty, P; Koller, H.; Mihovilovic, M. (1992). "Directed ortho lithiation of phenylcarbamic acid 1,1-dimethylethyl ester (N-BOC-aniline). Revision and improvements". Journal of Organic Chemistry. 57 (25): 6833–6837. doi:10.1021/jo00051a030.
- Fitt, J. J.; Gschwend, H. E. (1984). "Reaction of n-, sec-, and tert-butyllithium with dimethoxyethane (DME): a correction". Journal of Organic Chemistry. 49: 209–210. doi:10.1021/jo00175a056.
- Errington, R. M. (1997). Advanced practical inorganic and metalorganic chemistry (Google Books excerpt). London: Blackie Academic & Professional. pp. 47–48. ISBN 978-0-7514-0225-4.
- "Harran Lab: UCLA".
- Jyllian Kemsley (2009-01-22). "Researcher Dies After Lab Fire". Chemical & Engineering News.
- Jyllian Kemsley (2009-04-03). "Learning From UCLA: Details of the experiment that led to a researcher's death prompt evaluations of academic safety practices". Chemical & Engineering News.
- Los Angeles Times, 2009-03-01