Phenylpropene
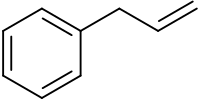
Phenylpropene is the organic compound with the formula C6H5CH2CH=CH2. It is a colorless liquid. The compound consists of a phenyl group attached to allyl. Phenylpropene isomerizes to trans-propenylbenzene.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Prop-2-en-1-yl)benzene | |
| Other names
Allylbenzene; 3-Phenyl-1-propene; 2-Propenylbenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.005.542 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10 | |
| Molar mass | 118.179 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.893 g/cm3 |
| Melting point | −40 °C (−40 °F; 233 K) |
| Boiling point | 156 °C (313 °F; 429 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In plant biochemistry, the phenylpropene skeleton is the parent (simplest representation) of the phenylpropanoids. Prominent derivatives include eugenol, safrole, and many others.[2]
References
- Hassam, Mohammad; Taher, Abu; Arnott, Gareth E.; Green, Ivan R.; van Otterlo, Willem A. L. (2015). "Isomerization of Allylbenzenes". Chemical Reviews. 115 (11): 5462–5569. doi:10.1021/acs.chemrev.5b00052. PMID 25993416.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Vogt, Thomas (2010). "Phenylpropanoid Biosynthesis". Molecular Plant. 3 (1): 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
External links
 Media related to Allylbenzene at Wikimedia Commons
Media related to Allylbenzene at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.