Fosravuconazole
Fosravuconazole (trade name Nailin) is a triazole antifungal agent.[1][2] In Japan, it is approved for the treatment of onychomycosis, a fungal infection of the nail.[3] It is a prodrug that is converted into ravuconazole.[1]
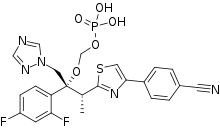 | |
| Clinical data | |
|---|---|
| Trade names | Nailin |
| Other names | BMS-379224; BFE-1224; E-1224 |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H20F2N5O5PS |
| Molar mass | 547.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The Drugs for Neglected Diseases Initiative and the Japanese pharmaceutical company Eisai are studying fosravuconazole as a potential treatment for eumycetoma.[1][4][5]
References
- Yamaguchi H (2016). "Potential of Ravuconazole and its Prodrugs as the New OralTherapeutics for Onychomycosis". Medical Mycology Journal. 57 (4): E93–E110. doi:10.3314/mmj.16-00006. PMID 27904057.
- "Fosravuconazole - Seren Pharmaceuticals". Adis Insight. Springer Nature Switzerland AG.
- "Oral Antifungal Agent Nailin Capsules 100 mg Approved in Japan" (Press release). Eisai. January 19, 2018.
- "Fosravuconazole". Drugs for Neglected Diseases Initiative.
- "Drugs for Neglected Diseases initiative and Eisai Co., Ltd. to Test Drug Candidate for Eumycetoma | News Release:2015 | Eisai Co., Ltd". www.eisai.com. Retrieved 2020-08-14.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.