Beryllium bromide
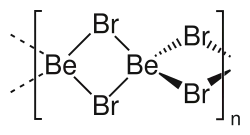
Beryllium bromide is the chemical compound with the formula BeBr2. It is very hygroscopic and dissolves well in water. The compound is a polymer with tetrahedral Be centres.[3]
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Beryllium bromide | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.196 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| BeBr2 | |
| Molar mass | 168.820 g/mol |
| Appearance | colorless white crystals |
| Density | 3.465 g/cm3 (20 °C) |
| Melting point | 508 °C (946 °F; 781 K)sublimes at 473 °C (883 °F; 746 K) |
| Boiling point | 520 °C (968 °F; 793 K)[1] |
| Highly[1] | |
| Solubility | soluble in ethanol, diethyl ether, pyridine insoluble in benzene |
| Structure | |
| Orthorhombic | |
| Thermochemistry | |
Heat capacity (C) |
0.4111 J/g K |
Std molar entropy (S |
9.5395 J/K |
Std enthalpy of formation (ΔfH⦵298) |
-2.094 kJ/g |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
see Berylliosis |
| GHS labelling: | |
   | |
| Danger | |
| H301, H315, H317, H319, H330, H335, H350i, H372, H411 | |
| P260, P301+P310, P304+P340, P305+P351+P338, P320, P330, P405, P501 | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[2] |
REL (Recommended) |
Ca C 0.0005 mg/m3 (as Be)[2] |
IDLH (Immediate danger) |
Ca [4 mg/m3 (as Be)][2] |
| Related compounds | |
Other anions |
Beryllium fluoride Beryllium chloride Beryllium iodide |
Other cations |
Magnesium bromide Calcium bromide Strontium bromide Barium bromide Radium bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation and reactions
It can be prepared by reacting beryllium metal with elemental bromine at temperatures of 500 °C to 700 °C:[1]
- Be + Br2 → BeBr2
Beryllium bromide is also formed when treating beryllium oxide with hydrobromic acid:
- BeO + 2 HBr → BeBr2 + H2O
It hydrolyzes slowly in water: BeBr2 + 2 H2O → 2 HBr + Be(OH)2
Structure
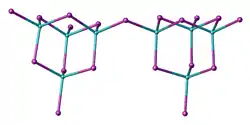
Two forms (polymorphs) of BeBr2 are known. Both structures consist tetrahedral Be2+ centers interconnected by doubly bridging bromide ligands. One form consist of edge-sharing polytetrahedra. The other form resembles zinc iodide with interconnected adamantane-like cages.[4]
Safety
Beryllium compounds are toxic if inhaled or ingested.
References
- Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, pp. 61–62, ISBN 0-8493-8671-3, retrieved 2007-12-10
- NIOSH Pocket Guide to Chemical Hazards. "#0054". National Institute for Occupational Safety and Health (NIOSH).
- Crystal modifications of Beryllium dihalides BeCl2, BeBr2, and BeI2 Troyanov, S. I. Zhurnal Neorganicheskoi Khimii (2000), 45(10), 1619-1624.
- Troyanov, S.I. (2000). "Crystal Modifications of Beryllium Dihalides BeCl2, BeBr2 and BeI2". Zhurnal Neorganicheskoi Khimii. 45: 1619-1624.
