Reactive nitrogen
Reactive nitrogen ("Nr") is a term used for a variety of nitrogen compounds that support growth directly or indirectly. Representative chemical species of Nr include the gases nitrogen oxides (NOx), ammonia (NH3), nitrous oxide (N2O), as well as the anion nitrate (NO3−). Most of these species are used in intensive farming . Nitrogen makes up about 75% of the atmosphere in an unreactive ("unfixed") form N2. Reactive nitrogen is "fixed" mainly by the microbes (eg., Bacteria and Archaea) of the soil that fix N2 into mainly NH3 but also other species. Legumes, a type of plant in the Fabacae family, are symbiants to some of these microbes that fix N2. NH3 is a building block to Amino acids and proteins amongst others things essential for life. However, in 1915 the Haber-Bosch process was invented. It uses fossil fuels to capture N2 and along with Hydrogen (H2) heats them up to 500 degree Celsius and under 250 bars of pressure fixes N2 that is then converted into fertilizers like Urea. This urea increases crop yields and has freed thousands of people from hunger. However, agriculture is under pressure to feed an extra 2 billion mouths by 2050 that they are using too much N fertilizers and it is polluting: leaching from soils into waterways, depleting oxygen (eutrophication), known as dead zones, in fresh and marine waters.[1] Nr is remains in the nitrogen cycle but undergoes Denitrification by mibrobes.

Reactive nitrogen compounds
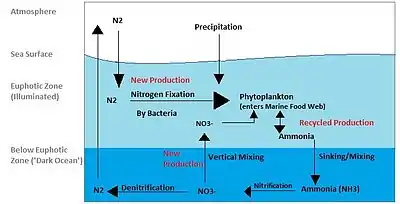
In the environmental context, reactive nitrogen compounds include the following classes:
- oxide gases: nitric oxide, nitrogen dioxide, nitrous oxide. Containing oxidized nitrogen, mainly the result of industrial processes and internal combustion engines.
- anions: nitrate, nitrite. Nitrate is a common component of fertilizers, e.g. ammonium nitrate.
- amine derivatives: ammonia and ammonium salts, urea. Containing reduced nitrogen, these compounds are components of fertilizers.
All of these compounds enter into the nitrogen cycle.
As a consequence, an excess of Nr can affect the environment relatively quickly. This also means that nitrogen-related problems need to be looked at in an integrated manner.[2]
References
- Citations
- Sutton, Mark A.; Bleeker, Albert (2013). "Environmental science: The shape of nitrogen to come". Nature. 494 (7438): 435–437. Bibcode:2013Natur.494..435S. doi:10.1038/nature11954. PMID 23426258. S2CID 4417543.
- http://international.vrom.nl/pagina.html?id=37594
- General references
- Hatfield, Jerry L; Follett, Ronald F (2008-07-16). Nitrogen in the environment: sources, problems, and management. The Scientific World Journal. Vol. 1 Suppl 2. Elsevier. pp. 920–6. doi:10.1100/tsw.2001.269. ISBN 978-0-12-374347-3. PMC 6084157. PMID 12805892.
- Braun, Elizabeth; Division Of Technology, United Nations Environment Programme; Industry; Economics; Hole, Woods Hole Research Center (Woods; ), Mass; Initiative, International Nitrogen (2007). Reactive nitrogen in the environment: too much or too little of a good thing. UNEP/Earthprint. ISBN 978-92-807-2783-8.