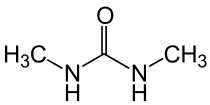
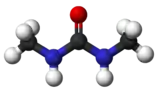
Dimethylurea
Dimethylurea (DMU) (IUPAC systematic name: 1,3-Dimethylurea ) is a urea derivative and used as an intermediate in organic synthesis. It is a colorless crystalline powder with little toxicity.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N′-Dimethylurea | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.272 |
| EC Number |
|
| KEGG | |
| MeSH | 1,3-dimethylurea |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H8N2O | |
| Molar mass | 88.110 g·mol−1 |
| Appearance | Colorless, waxy crystals |
| Odor | Odorless |
| Density | 1.142 g mL−1 |
| Melting point | 104.4 °C; 219.8 °F; 377.5 K |
| Boiling point | 269.1 °C; 516.3 °F; 542.2 K |
| 765 g L−1 | |
| -55.1·10−6 cm3/mol | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
−312.1–−312.1 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−2.0145–−2.0089 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
| H373 | |
| P260, P314, P501 | |
| Flash point | 157 °C (315 °F; 430 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
4 g kg−1 (oral, rat) |
| Related compounds | |
Related ureas |
Carmustine |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uses
1,3-Dimethylurea is used for synthesis of caffeine, theophylline, pharmachemicals, textile aids, herbicides and others.[1] In the textile processing industry 1,3-dimethylurea is used as intermediate for the production of formaldehyde-free easy-care finishing agents for textiles. The estimated world production of DMU is estimated to be less than 25,000 tons.
References
- http://www.inchem.org/documents/sids/sids/96311.pdf SIDS Initial Assessment Report
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.