Cyclopentadienyl nickel nitrosyl
Cyclopentadienyl nickel nitrosyl is a highly toxic organonickel chemical. In its pure form, it is a diamagnetic, volatile, relatively air-stable liquid with a blood-red color. It has been reported to be the simplest mono-cyclopentadienyl metal complex.[2] The chemical was discovered in 1954 by a team at The International Nickel Company.[3] The molecular formula is (C5H5)NiNO. It can be prepared by treating nickelocene with nitric acid.[4] It is extremely toxic (T+), and is considered to be one of the most poisonous organometallic chemicals ever developed. Its toxicity is said to be comparable to nickel tetracarbonyl.[2]
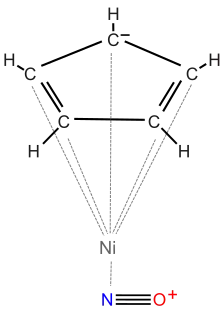 | |
| Names | |
|---|---|
| IUPAC name
azanylidyneoxidanium;cyclopenta-1,3-diene;nickel | |
| Other names
Cyclopentadienylnickelnitrosyl (6CI); Nickel, nitrosylcyclopentadienyl- (7CI); | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| (C5H5)NiNO | |
| Molar mass | 153.7927 g/mol |
| Appearance | Blood-red liquid |
| Odor | Unpleasant, disagreeable[1] |
| Melting point | −41 °C (−42 °F; 232 K) |
| Boiling point | 144–145 °C (291–293 °F; 417–418 K) |
| Insoluble[1] | |
| Solubility | Very soluble in all organic compounds |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Extremely Toxic (T+) |
| GHS labelling: | |
   | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Due to its high toxicity, cyclopentadienyl nickel nitrosyl has very limited usage. It was patented as a fuel additive and anti-caking agent, but it was never used for these purposes due to the health hazards it posed.[2] In the past, it was also studied for its spectroscopic qualities, and saw limited use as a catalyst in organic chemical reactions, but it has since been discounted in favor of less toxic compounds.
Notes
- Herrmann, Wolfgang A. (2014-05-14). Synthetic Methods of Organometallic and Inorganic Chemistry, Volume 8, 1997: Volume 8: Transition Metals. p. 89. ISBN 9783131792419.
- Jolly, P. W. (2012-12-02). The Organic Chemistry of Nickel: Organonickel Complexes. p. 464. ISBN 9780323146906.
- US Patent 3088959 – Process of making cyclopentadienyl nickel nitrosyl compounds
- Pauling, Linus (1960). The nature of the chemical bond and the structure of molecules and crystals : an introduction to modern structural chemistry. Ithaca, N.Y: Cornell University Press. ISBN 978-0-8014-0333-0.
