Freshwater acidification
Freshwater acidification occurs when acidic inputs enter a body of fresh water through the weathering of rocks, invasion of acidifying gas (e.g. carbon dioxide), or by the reduction of acid anions, like sulfate and nitrate within the lake.[1] Freshwater acidification is primarily caused by sulfur oxides (SOx) and nitrogen oxides (NOx) entering the water from atmospheric depositions and soil leaching.[1] Runoff that contains these compounds may be accompanied by acidifying hydrogen ions and inorganic aluminum, which can be toxic to marine organisms. Acid rain is also a contributor to freshwater acidification. It is created when SOx and NOx react with water, oxygen, and other oxidants within the clouds.[2]

The buffering capacity of soils and bedrocks within the freshwater ecosystem can contribute to the acidity of the water. Each freshwater reservoir has a capacity to resist changes in pH, but an excess input of acids into the reservoir can cause the buffering capacity to decrease, eventually causing the water to become more acidic.[1] An increase in atmospheric CO2 affects freshwater acidity as more of it dissolves into water, the more acidic it becomes.[3] It is difficult to quantify the effects of anthropogenic CO2 due to the various carbon fluxes in freshwater ecosystems.[4] Rising freshwater acidification is harmful to various aquatic organisms.
Freshwater vs. ocean acidification
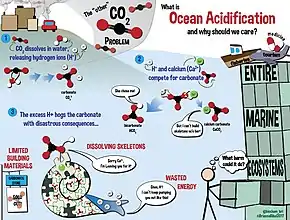
The ocean and the atmosphere are constantly exchanging massive amounts of CO2.[5] Over the last 800,000 years, the concentration of CO2 in the atmosphere has remained around 172-300 parts per million by volume (ppmv).[5] With increasing anthropogenic CO2 emissions recently, this number has increased to 387 ppmv in 2009.[5] From 2000-2008, 26% of anthropogenic CO2 was absorbed by the ocean.[5] CO2 is the primary factor affecting ocean pH, though other factors also play a role.[5] When dissolved in water, CO2 acts as a weak acid that primarily affects carbonate chemistry.[5] Dissolved CO2 increases the concentration of bicarbonate ions (HCO3−), dissolved inorganic carbon (CT) and lowers the pH.[5] Similar to oceans, freshwater bodies also absorb atmospheric CO2, lowering the pH.[6] In addition to CO2, freshwater reservoir's pH values are altered by acid rain, nutrient runoff, and anthropogenic pollutants.[6] Freshwater takes up CO2 in the same mechanism as seawater; however, freshwater alkalinity is much lower than seawater, due to the absence of a salt-buffer.[6] Without this salt-buffer, pH changes in freshwater tend to be more evident than in ocean water. In freshwater systems, newly released H+ ions are not buffered by as many bicarbonate (HCO3−) ions as ocean water. Therefore, freshwater biota tends to have a higher evolutionary pH tolerance than seawater biota.
Causes
CO2
Carbon dioxide reacts with water to form carbonic acid, bicarbonate, carbonate, and acidic protons via the following equilibria:
CO2 (aq) + H2O ⇌ H2CO3 ⇌ HCO3− + H+ ⇌ CO32− + 2 H+
Dissociation of carbonic acid decreases the pH of the solution. The degree of dissociation is controlled by the overall chemistry of the solution; in particular, alkalinity and temperature are primary controlling factors. There has been a clear increase of pCO2 in some freshwater ecosystems in the last century due to anthropogenic influence that is contributing to freshwater acidification.[5] It is often difficult to quantify the effects of pCO2 (partial pressure) in freshwater due to the various sources of carbon dioxide and the many factors that affect it such as the surrounding landscape, climate, the organisms present, the water's chemistry, and biological processes (e.g. photosynthesis, respiration).[6] The dominant species of inorganic carbon present in freshwater can be a pH indicator with more CO32- being present in basic water and free CO2 in acidic water because when CO2 dissolves into the surface of freshwater it reacts to form carbonic acid.[6] Along with the overall trend of increasing CO2 in the atmosphere that is being absorbed by bodies of water, the levels of carbon dioxide fluctuate daily and seasonally.[7]
SOx and NOx
Two of the main contributors to freshwater acidification are sulfur oxides and nitric oxides. The accelerated burning of fossil fuels over the past two centuries has largely contributed to the acidification of freshwater ecosystems. International cooperation and environmental legislation have reduced SOx and NOx in recent decades as sulfate emissions peaked in the 1970s with nitrogen following behind 10 years later.[8] Increased sulfate concentration in runoff due to increased acidity inputs is coupled with both an increase in base cation run-off and a decrease of bicarbonate, creating the acidifying effects in aquatic systems.[9] Acidic rain seeps into and reacts with clay particles in the soil which leads to the leaching of aluminum into nearby bodies of water. Thus as the pH levels decrease, aluminum levels will increase. The higher levels of aluminum can also contaminate drinking water for humans which can lead to several health diseases.[10] This creates a toxic environment to marine species and their environment which can lead to the extinction of species, reductions in population size, and overall a decrease in biodiversity. Most nitrogen in its natural state that is put into terrestrial ecosystems will be utilized by vegetation. However, in large amounts, not all of the nitrogen is able to be taken up by vegetation so the excess gets washed away with runoff in the form of nitrate. Nitrate will contribute to acidification in the same manner as sulfate.
Buffering capacity
The buffering capacity of ecosystems help them resist changes in pH and when a system lacks this, it can lead to the acidification of its freshwater. For example, Atlantic Canada has the lowest acid deposition rates in Eastern North America yet has the most acidic waters on the continent.[11] This is due to the low buffering capacity of the regional bedrock and the addition of natural organic acids produced by close by wetlands.[11] Specifically, in Southwestern and Eastern Nova Scotia, there is a combination of high organic acidity, poor buffering, and high acid deposition to produce a very low surface water pH and acid neutralization capacity (ANC) values.[11] In most of the Atlantic region, granite and shale bedrock are found, which contain very little buffering material.[11] Soil formed from low-buffering materials and the waters that drain from them are, therefore, susceptible to acidification, even under low acid deposition.[11] Some species are able to withstand low pH levels in their environment. For example, frogs and perches can withstand a pH level of 4. This allows these species to be unaffected to the acid deposition in their aquatic environment, allowing them to survive in these conditions. However, most aquatic species such as clams and snails are unable to withstand low pH levels which negatively impacts their growth and survival. The high acidic levels deteriorate their thick shells which are not good for their protection from predators.[12]
Harmful effects on aquatic ecosystems
%252C_Veenpluis_(Eriophorum_angustifolium)_03.JPG.webp)
Acidification of freshwater ecosystems may have significant negative effects on these ecosystems. Changes in pH as a result of freshwater acidification imposes physiological challenges on individual organisms, may decrease native biodiversity, and can alter ecosystem structure and function entirely. Macro-invertebrates and large vertebrates alike are particularly sensitive to acidification; these species exhibit higher mortality and lower reproductive rates under acidified conditions.[9] These species are forced expend more energy on buffering of their body conditions to retain a livable pH, and therefore must limit energy expenditure on processes such as hunting, sheltering, and reproducing.[9] Embryonic development, and therefore species success, is also compromised in acidified freshwaters.[9] Conversely, algae becomes far more successful in acidified environments, and may quickly dominate these habitats, outcompeting other species.
In most acidic freshwater reservoirs, there will be an increase in the development of mosses and algae.[9] In particular, it is common to see an increase in the abundance of the moss sphagnum. Sphagnum has a high capacity to exchange H+ for basic cations within freshwater. The thick layer of sphagnum restricts the exchange between surface water and sediment, further contributing to reduction in nutrient cycling in the ecosystem.
Reducing Acidification
There are processes that can remediate the acidification of freshwaters. Liming is one such practice where calcium carbonate (CaCO3) is added to these systems. When added to rivers, in some, there were positive effects on the wildlife, increasing the abundance of fish and acid-sensitive invertebrates.[13] However, these effects are variable and other studies had results that showed a decrease in invertebrate abundance. A large decrease of acid rain and acidic bodies of water in the past couple of decades has been a direct result of governmental regulations on anthropogenic emissions, specifically SOx and NOx.[14]
References
- Psenner, Roland (March 1994). "Environmental impacts on freshwaters: acidification as a global problem". Science of the Total Environment. 143 (1): 53–61. Bibcode:1994ScTEn.143...53P. doi:10.1016/0048-9697(94)90532-0. ISSN 0048-9697.
- Irwin, J.G.; Williams, M.L. (1988). "Acid rain: Chemistry and transport". Environmental Pollution. 50 (1–2): 29–59. doi:10.1016/0269-7491(88)90184-4. ISSN 0269-7491. PMID 15092652.
- Jean-Pierre Gattuso; Lina Hansson, eds. (2011). Ocean acidification. Oxford University Press. ISBN 9780199591084. OCLC 975179973.
- "Measurements and observations : OCB-OA". Whoi.edu. Retrieved 2019-03-24.
- Weiss, Linda C.; Pötter, Leonie; Steiger, Annika; Kruppert, Sebastian; Frost, Uwe; Tollrian, Ralph (January 2018). "Rising pCO2 in Freshwater Ecosystems Has the Potential to Negatively Affect Predator-Induced Defenses in Daphnia". Current Biology. 28 (2): 327–332.e3. doi:10.1016/j.cub.2017.12.022. ISSN 0960-9822. PMID 29337079.
- Hasler, Caleb T.; Butman, David; Jeffrey, Jennifer D.; Suski, Cory D. (January 2016). Sterner, Robert (ed.). "Freshwater biota and rising pCO 2 ?". Ecology Letters. 19 (1): 98–108. doi:10.1111/ele.12549. PMID 26610406.
- Muniz, Ivar P. (1990). "Freshwater acidification: its effects on species and communities of freshwater microbes, plants and animals". Proceedings of the Royal Society of Edinburgh, Section B: Biological Sciences. 97: 227–254. doi:10.1017/s0269727000005364. ISSN 0269-7270.
- Cardoso, A.C.; Free, G.; Nõges, P.; Kaste, Ø.; Poikane, S.; Solheim, A. Lyche (2009). "Lake Management, Criteria". Encyclopedia of Inland Waters. Elsevier. pp. 310–331. doi:10.1016/b978-012370626-3.00244-1. ISBN 9780123706263.
- Henriksen, Arne; Kämäri, Juha; Posch, Maximilian; Wilander, Anders (1992). "Critical Loads of Acidity: Nordic Surface Waters". Ambio. 21 (5): 356–363. ISSN 0044-7447. JSTOR 4313961.
- "Acid Rain, Aluminum Link Found". Washington Post. Retrieved 19 April 2022.
- Clair, Thomas A.; Dennis, Ian F.; Scruton, David A.; Gilliss, Mallory (December 2007). "Freshwater acidification research in Atlantic Canada: a review of results and predictions for the future". Environmental Reviews. 15 (NA): 153–167. doi:10.1139/a07-004. ISSN 1181-8700.
- "Effects of Acid Rain - Surface Waters and Aquatic Animals" (PDF). Landuse.alberta.ca. Retrieved 19 April 2022.
- Mant, Rebecca C.; Jones, David L.; Reynolds, Brian; Ormerod, Steve J.; Pullin, Andrew S. (2013-08-01). "A systematic review of the effectiveness of liming to mitigate impacts of river acidification on fish and macro-invertebrates". Environmental Pollution. 179: 285–293. doi:10.1016/j.envpol.2013.04.019. ISSN 0269-7491.
- Menz, Fredric C.; Seip, Hans M. (2004-08-01). "Acid rain in Europe and the United States: an update". Environmental Science & Policy. 7 (4): 253–265. doi:10.1016/j.envsci.2004.05.005. ISSN 1462-9011.